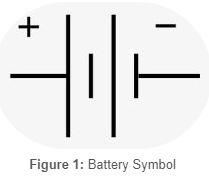
Understanding Batteries: A Comprehensive Guide
In today’s interconnected world, batteries are the silent champions powering our daily lives, seamlessly facilitating everything from our smartphones’ endless scrolling sessions to the emission-free journeys of electric vehicles. Yet, behind this ubiquitous presence lies a diverse array of battery types, each tailored to specific applications and bearing unique characteristics. From the reliable alkaline cells in our TV remote to the high-energy lithium-ion packs propelling the electric revolution, batteries come in various shapes, sizes, and chemistries, each with its own set of advantages and limitations. However, amidst their convenience and versatility, understanding the fundamentals of batteries becomes paramount for ensuring not just efficient usage but also safety in handling and disposal. Whether it’s optimizing device runtime or mitigating potential hazards, delving into the intricacies of batteries unveils a world where knowledge becomes power – quite literally. Thus, embarking on this journey to unravel the mysteries of batteries is not merely an exercise of curiosity but a crucial step towards harnessing their full potential while safeguarding ourselves and our environment.
What is a Battery?
A battery is a device that converts stored chemical energy into electrical energy through a series of electrochemical reactions. It typically consists of one or more electrochemical cells, each containing an anode, a cathode, and an electrolyte.
Basic Components of a Battery
- Anode: The anode is the electrode where oxidation (loss of electrons) occurs during discharge. It releases electrons into the external circuit.
- Cathode: The cathode is the electrode where reduction (gain of electrons) occurs during discharge. It accepts electrons from the external circuit.
- Electrolyte: The electrolyte is a medium that allows ions to move between the anode and cathode, completing the electrical circuit. It can be liquid, gel, or solid, depending on the type of battery.
How Batteries Generate Electricity
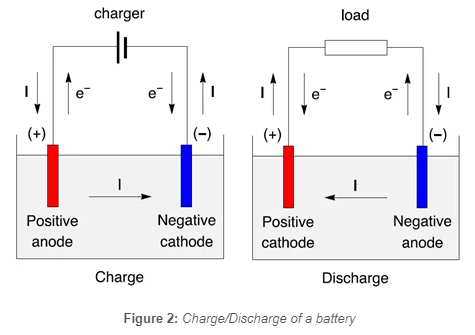
When a battery is connected to a circuit, chemical reactions occur at the anode and cathode. At the anode, oxidation reactions release electrons, creating an electron surplus. These electrons flow through the external circuit to the cathode, where reduction reactions accept the electrons. The movement of electrons generates an electric current that can power electronic devices.
The potential difference between the positive and negative terminals increases during a battery charge cycle. Consequently, the anode becomes positively charged, and the cathode becomes negatively charged.
While this potential difference decreases during the discharge cycle, thus the anode becomes negatively charged, and the cathode becomes positively charged.
Figure 2 shows the charge and discharge of a secondary battery represented as an electrochemical cell, with electrons and current directions.
Types of Batteries
Batteries are mainly divided into the following two types:
1. Primary (Non-rechargeable) Batteries
Primary batteries are designed for one-time use. Once the chemical reactions are depleted, they cannot be recharged. Common examples include zinc-carbon, alkaline, and lithium-metal batteries.
2. Secondary (Rechargeable) Batteries
Secondary batteries, also known as rechargeable batteries, can be recharged multiple times by applying an external electrical current that reverses the chemical reactions. Examples include lithium-ion (Li-ion), Nickel-cadmium (NiCd), nickel-metal hydride (NiMH), and lead-acid batteries.
Overview of Common Battery Types
- Zinc-Carbon: Zinc-carbon are one of the earliest and least expensive primary batteries. These batteries utilize zinc as the anode and manganese dioxide as the cathode, with an electrolyte containing ammonium chloride or zinc chloride. They are commonly used in low-drain devices such as remote controls, flashlights, and wall clocks due to their affordability and reliability for moderate power needs.
- Alkaline: The alkaline battery is an improved version of the zinc-carbon battery. Alkaline delivers more energy at higher load currents than zinc-carbon. They are widely used in household electronics due to their long shelf life and relatively low cost.
- Lithium-Metal: Lithium-metal batteries employ lithium metal as the anode and various lithium compounds as the cathode. They are used in applications demanding high energy density and compact size, such as in electric vehicles, portable electronics, and grid energy storage systems.
- Lithium-Ion: Li-ion batteries employ lithium compounds as electrodes and an organic electrolyte. They offer high energy density, and lightweight construction, and are commonly used in portable electronic devices like smartphones and laptops.
- Lead-Acid: Lead-acid batteries utilize lead dioxide and lead as electrodes with a sulfuric acid electrolyte. They are commonly found in automotive applications due to their ability to deliver high currents.
- Nickel-Cadmium: Nickel-cadmium (NiCd) batteries feature nickel hydroxide as the positive electrode and cadmium as the negative electrode, with a potassium hydroxide electrolyte. NiCd are known for their durability and are commonly used in applications requiring high discharge currents and long service life, such as power tools, medical devices, and aviation.
- Nickel-Metal-Hydride: Nickel-metal-hydride (NiMH) batteries utilize a metal hydride alloy as the positive electrode and nickel oxyhydroxide as the negative electrode, with a potassium hydroxide electrolyte. NiMH, serving as a more environmentally friendly alternative to NiCd, are commonly used in hybrid cars, medical instruments, and consumer electronics like AA and AAA cells for various portable devices.
Pros and Cons of Each Battery Type
| Types of Batteries | Advantages | Disadvantages |
| Zinc-carbon | – Inexpensive – Widely available | – Low energy density – Shorter lifespan compared to other types – Poor performance in high-drain devices |
| Alkaline | – Higher energy density than zinc-carbon batteries – Longer shelf life – Suitable for a wide range of devices | – Relatively higher cost compared to zinc-carbon – Performance may degrade at low temperatures – Disposal can be environmentally harmful |
| Lithium-metal | – High energy density, making them lightweight and compact – Long shelf life – Suitable for high-drain devices like digital cameras | – Prone to overheating and fire if damaged or improperly charged – More expensive than alkaline or zinc-carbon batteries – Limited recycling infrastructure |
| Lithium-Ion | – High energy density – Lightweight and compact – Rechargeable, reducing waste | – More expensive upfront cost – Aging and capacity degradation over time, especially with frequent charging – Risk of thermal runaway if damaged or exposed to high temperatures |
| Lead-Acid | – Low cost – Well-established technology – Suitable for high-current applications like starting engines | – Heavy and bulky – Low energy density – Susceptible to sulfation if not maintained properly |
| Nickel-cadmium | – Robust and durable – High discharge rates – Long lifespan with proper care | – Contains toxic cadmium, harmful to the environment – Lower energy density compared to newer battery types – Memory effect if not fully discharged before recharging |
| Nickel-metal hydride | – Environmentally friendly, no toxic heavy metals – Higher energy density than NiCd batteries – Less prone to memory effect | – Self-discharge rate is higher compared to other rechargeable types – Sensitive to high temperatures, reducing lifespan – The initial cost is higher than NiCd batteries |
Battery Capacity and Battery Voltage
Battery Capacity
The battery capacity refers to the amount of charge a battery can store and is typically measured in milliampere-hours (mAh) or ampere-hours (Ah), depending on the size of the battery. It indicates how long a battery can sustain a specific current before it needs recharging.
milliampere-hours (mAh) / milliamperes (mA) = Hours (h)
- Example: A battery with a capacity of 3000mAh can theoretically provide a current of 3000 milliamperes for one hour or 300 milliamperes for 10 hours.
Battery Voltage
The voltage output of a battery cell is determined by the specific chemistry used inside. For instance, Alkaline cells consistently provide a voltage of 1.5V, lead-acid cells typically offer 2V, and lithium cells deliver 3V. Since batteries can be constructed using multiple cells, configurations vary. Consequently, it’s uncommon to encounter a standalone 2V lead-acid battery. Instead, these cells are often interconnected within a battery casing to create configurations such as 6V, 12V, or 24V.
Relationship between Capacity and Voltage of a Battery
This battery power is usually measured in Watt-hours (Wh). A Watt-hour is found by multiplying the voltage (V) of the battery by how much current (Amps) it can provide for a certain period, typically in hours. So,
Voltage * Amps * hours = VAh = Wh
Because the voltage remains mostly the same for a type of battery due to its internal chemistry (like alkaline, lithium, lead acid, etc.), sometimes only the Amps*hour measurement is shown, written as Ah or mAh. To find Wh, multiply the Ah by the standard voltage.
- Example: If we have a 2V standard battery with a capacity of 1 Amp-hour, then it has a capacity of 2 Wh. Having 1 Ah means that theoretically, we can draw 1 Amp of current for one hour, or 0.1A for 10 hours, or 10 mA for 100 hours.
Battery capacity and voltage are related but not directly proportional. While a battery’s voltage remains relatively constant throughout most of its discharge cycle, its capacity gradually decreases as the stored energy is depleted.
Factors Affecting Battery Capacity
Battery capacity effecting factors refer to the various elements that influence the amount of energy a battery can store and deliver at any given time. These factors include:
- Battery Chemistry
- Battery Temperature
- Charge and discharge rates
- Depth of discharge
- Overcharging and undercharging
- State of charge (SoC)
- Age of the battery
- Electrode design and materials
- Internal resistance
- Electrolyte composition
- Cell design and construction
- Manufacturing quality
- Cycling conditions
- Environmental conditions
- Voltage limits
Battery Lifespan and Cycle Life
Battery lifespan refers to the duration of time a battery remains functional before it needs replacement due to degradation or wear. Cycle life refers to the number of charge-discharge cycles a battery can undergo before its capacity significantly diminishes.
Factors Influencing Battery Lifespan
Several factors influence the lifespan of a battery:
- Chemistry: The type of battery chemistry used (such as lithium-ion, lead-acid, nickel-cadmium) significantly affects its lifespan.
- Temperature: Extreme temperatures, both hot and cold, can degrade battery performance and shorten lifespan.
- Charge and Discharge Cycles: The number of times a battery is charged and discharged affects its longevity.
- Depth of Discharge: Fully discharging a battery frequently can reduce its lifespan compared to shallow discharges.
- Overcharging and Undercharging: Excessive charging or discharging beyond recommended levels can damage a battery and shorten its lifespan.
- Storage Conditions: Storing battery in improper conditions, such as high temperatures or high humidity, can lead to degradation over time.
- Quality of Manufacturing: The quality of materials and manufacturing processes used to create the battery can impact its lifespan.
- Usage Patterns: Heavy usage or rapid charging can put additional stress on the battery, affecting its lifespan.
- Maintenance: Neglecting regular maintenance, such as failing to clean battery terminals or provide adequate ventilation, can lead to a reduction in the battery’s lifespan.
- Age: As batteries age, their internal components degrade, leading to reduced performance and lifespan.
How to Extend Battery Lifespan
The lifespan of a battery can be improved through the following practices:
- Minimize high discharge rates to avoid additional stress on the battery
- Avoid leaving batteries plugged in for extended periods after they are fully charged
- Avoid overcharging or completely draining batteries frequently. Instead, aim for partial discharge cycles
- Clean battery terminals periodically to prevent corrosion, and ensure proper ventilation to prevent overheating
- Use chargers recommended by the battery manufacturer to ensure proper charging rates and prevent overcharging
- Keep batteries away from excessive heat or cold, as extreme temperatures can degrade their performance and longevity
- Store batteries in a cool, dry place away from direct sunlight and moisture. Ensure they are stored at around 50% charge if they will be unused for an extended period
- Adhere to the manufacturer’s recommendations for usage, maintenance, and charging practices to maximize battery lifespan
Battery Safety
Handling batteries safely is crucial to prevent accidents, including leakage, overheating, and explosion, which can result from misuse or improper handling.
Common Safety Tips
Below are some essential battery safety tips to keep in mind:
- Use the Right Charger: Always use chargers recommended by the manufacturer to avoid overcharging or damaging the battery.
- Avoid Extreme Temperatures: Keep batteries away from excessive heat or cold, as extreme temperatures can affect their performance and safety.
- Inspect for Damage: Regularly check batteries for signs of damage such as leaks, bulges, or corrosion, and replace damaged batteries immediately.
- Charge Safely: Charge batteries on a stable, flat surface away from flammable materials, and never leave them unattended while charging.
- Store Properly: Store batteries in a cool, dry place away from direct sunlight and moisture, and keep them out of reach of children and pets.
- Handle with Care: Avoid dropping or mishandling batteries, as physical damage can lead to internal damage or leakage.
- Dispose Properly: Dispose of batteries according to local regulations or recycling programs to prevent environmental contamination.
- Don’t Mix: Avoid mixing old and new batteries, or different types of batteries, as this can lead to uneven charging and potential safety hazards.
- Use Protective Cases: When carrying batteries, use protective cases or covers to prevent short circuits or damage.
- Educate Yourself: Learn about the specific safety guidelines for the type of batteries you’re using, and follow manufacturer recommendations for usage and maintenance.
Risks Associated With Improper Battery Handling
Improper battery handling can pose various risks, ranging from minor inconveniences to serious safety hazards. Mishandling batteries, such as using incorrect chargers, exposing them to extreme temperatures, or damaging them through physical impact, can result in leaks, fires, or even explosions. Overcharging or discharging batteries beyond their recommended limits can cause thermal runaway, leading to the release of hazardous chemicals and potentially causing burns or property damage. Additionally, mixing different battery types or failing to dispose of batteries properly can pose environmental risks. Therefore, it is crucial to understand and adhere to proper battery handling procedures to minimize these risks and ensure both personal safety and environmental protection.
Battery Maintenance
Tips for Maintaining Batteries in Good Condition
- Keep batteries charged to prevent self-discharge and sulfation
- Store batteries in a cool, dry place away from extreme temperatures
- Ensure batteries are used for their intended purpose and incompatible devices
- Follow manufacturer charging guidelines to avoid overcharging and over-discharging
- Use batteries correctly incompatible devices, and avoid mixing old and new batteries
- Regularly clean battery terminals to prevent corrosion, and inspect for damage or leaks
- Prevent batteries from reaching critically low voltage levels, as this can lead to irreversible damage
- For rechargeable batteries, periodically exercise them by fully charging and discharging them to prevent capacity loss
- Invest in batteries with built-in protection circuits or use external battery management systems to monitor and maintain battery health
How to Store Batteries Properly for Long-Term Use
To store batteries properly for long-term use, it’s essential to follow a few key guidelines. When storing, remove the battery from the equipment and place it in a dry and cool place away from direct sunlight and moisture, as exposure to extreme temperatures or humidity can degrade battery performance. Avoid freezing, as batteries freeze more easily if kept discharged. Ideally, batteries should be stored at around 50% charge if they will be unused for an extended period, as storing them at full charge or completely discharged can lead to capacity loss and reduced lifespan. Additionally, consider using dedicated storage containers or organizers to keep batteries organized and protected from physical damage. Regularly inspect stored batteries for signs of damage or leaks, and replace any damaged batteries promptly. By adhering to these storage practices, you can help maintain the condition and performance of your batteries for long-term use.
Importance of Regular Maintenance for Rechargeable Batteries
Regular maintenance is crucial for ensuring optimal performance and longevity of rechargeable batteries. By conducting routine maintenance tasks such as cleaning battery terminals, monitoring charging habits, and inspecting for signs of wear or damage, users can prevent common issues such as corrosion, overcharging, or physical damage that can degrade battery performance over time. Additionally, regular maintenance helps identify potential problems early on, allowing for timely intervention and prevention of more serious issues. Proper maintenance not only extends the lifespan of rechargeable batteries but also ensures reliable and consistent performance, ultimately maximizing their value and utility for the user.
Future of Batteries
Emerging Battery Technologies
Emerging battery technologies encompass a variety of innovative approaches aimed at enhancing energy storage capabilities, improving efficiency, and addressing environmental concerns. Some of the notable emerging battery technologies include:
- Solid-State Batteries: These batteries use solid electrolytes instead of liquid or gel electrolytes found in traditional lithium-ion batteries, offering higher energy density, improved safety, and potentially faster charging rates.
- Lithium-Sulfur Batteries: Lithium-sulfur-batteries have the potential for higher energy density compared to lithium-ion batteries, which could result in longer-lasting and lighter-weight battery packs.
- Sodium-Ion Batteries: Sodium-ion-batteries utilize sodium ions instead of lithium ions, offering a potentially lower-cost alternative to lithium-ion-batteries while maintaining decent energy density and safety.
- Flow Batteries: Flow batteries store energy in external tanks of electrolyte solutions, allowing for scalable and customizable energy storage solutions suitable for grid-scale applications.
- Lithium-Air Batteries: Lithium-air-batteries have the potential for significantly higher energy density compared to lithium-ion-batteries, although challenges such as limited cycle life and stability still need to be addressed.
- Graphene-Based Batteries: Batteries incorporating graphene, a form of carbon with exceptional conductivity and strength, offer the potential for faster charging rates, longer lifespan, and improved energy storage capacity.
- Aluminum-Ion Batteries: Aluminum-ion batteries are being explored as a potential alternative to lithium-ion batteries, offering advantages such as faster charging rates, lower cost, and higher safety.
- Magnesium Batteries: Magnesium batteries utilize magnesium ions as the charge carriers, offering the potential for higher energy density compared to lithium-ion and utilizing more abundant and environmentally friendly materials.
These emerging battery technologies hold promise for revolutionizing energy storage across various applications, from consumer electronics to electric vehicles and grid-scale energy storage systems. While many of these technologies are still in the research and development phase, ongoing advancements are driving them closer to commercial viability and widespread adoption.
Potential Impact of Battery Advancements
The battery advancements are profound and far-reaching, with implications spanning various industries and societal sectors. Improved battery technologies promise to revolutionize the way we store and utilize energy, leading to advancements in electric vehicles, renewable energy integration, grid-scale energy storage, and portable electronics. With higher energy densities, faster charging rates, and improved safety features, advanced batteries can accelerate the transition to a cleaner and more sustainable energy future by reducing reliance on fossil fuels and mitigating greenhouse gas emissions. Additionally, advancements in battery technology can spur innovation and economic growth, creating new opportunities for job creation, investment, and technological leadership. As battery technologies continue to evolve and mature, their impact on transportation, energy infrastructure, and global sustainability efforts is expected to be transformative, driving toward a more efficient, resilient, and environmentally responsible energy ecosystem.
Outlook for the Future of Batteries
The future of batteries is incredibly promising, with ongoing research and development efforts driving innovation and advancement in energy storage technology. As demand for clean energy solutions grows and the electrification of transportation accelerates, batteries are poised to play a central role in enabling a more sustainable and efficient energy landscape. Continued improvements in battery performance, including higher energy densities, faster-charging rates, and longer lifespans, will unlock new possibilities for electric vehicles, renewable energy integration, and grid-scale energy storage applications. Moreover, the convergence of battery technology with emerging trends such as artificial intelligence, the Internet of Things (IoT), and smart grid technologies will further enhance the capabilities and versatility of batteries, enabling more intelligent and interconnected energy systems. With ongoing investments, collaboration, and innovation, the future of batteries holds immense potential to reshape the way we generate, store, and consume energy, paving the way toward a cleaner, more resilient, and sustainable energy future.
Conclusion
In conclusion, batteries are at the forefront of a transformative energy revolution, with advancements in technology poised to reshape industries and societal norms. From powering electric vehicles to storing renewable energy and enabling portable electronics, batteries play a pivotal role in driving progress towards a cleaner, more sustainable future. As research and development efforts continue to push the boundaries of innovation, the outlook for battery technology remains incredibly promising. With ongoing investments, collaboration, and commitment to sustainability, batteries have the potential to revolutionize the way we generate, store, and utilize energy, ushering in a new era of efficiency, resilience, and environmental responsibility. As we embrace the opportunities presented by battery advancements, we embark on a journey toward a brighter and more sustainable energy future for generations to come.
References
- Ada, l. (2024). All About Batteries. Retrieved from Adafruit Learning System: https://learn.adafruit.com/all-about-batteries/
- Cowie, I. (2013). All About Batteries. Retrieved from EE Times: https://www.eetimes.com/all-about-batteries-part-1-introduction/
- Kensington, S. (2023). Everything You Need To Know About Batteries. Retrieved from Get Home Schooled: https://gethomeschooled.com/everything-you-need-to-know-about-batteries/
- Learn About Batteries. (n.d.). Retrieved from Battery University: https://batteryuniversity.com/articles
- Sahapatsombut, U. (n.d.). Everything You Need to Know about Batteries. Retrieved from ENTEC: https://www.entec.or.th/knowledge-everything-you-need-to-know-about-batteries/

A dedicated Electrical Engineer with expertise in ISO auditing and a strong passion for sharing insights into the electrifying world of design. With over 5 years of diverse experience, I’ve powered through projects ranging from the manufacturing industry to building construction. My skillset extends to automation building design, where my meticulous electrical drawings ensure precision and attention to detail.